Table of Contents
Adding a Custom Consent Form to an Account
 Updated
by Billy Dowell
Updated
by Billy Dowell
Adding consent forms to your account
If you have additional legal requirements for participating in your studies, you can add custom consent forms or NDAs to your account to use for your studies.

Once attached, candidates will have to view and sign your document before being able to book a time, complete a survey, or start a task.
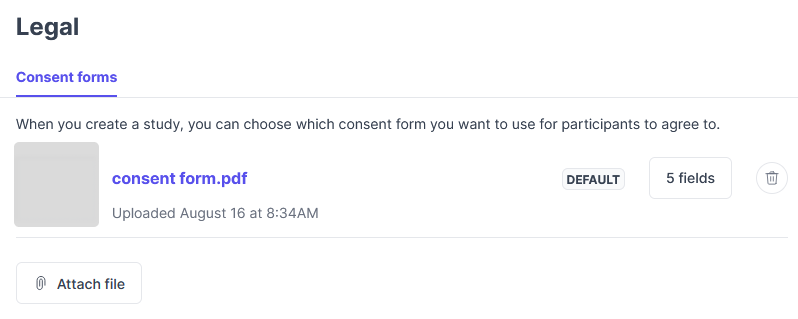
How to add a consent form
- In account settings, click Legal.
- Click Attach file.
- Attach your consent form (must be a PDF).
- Optional: Click Set as default to make the custom consent form the default for all studies.
- Optional: Change the transfer settings.
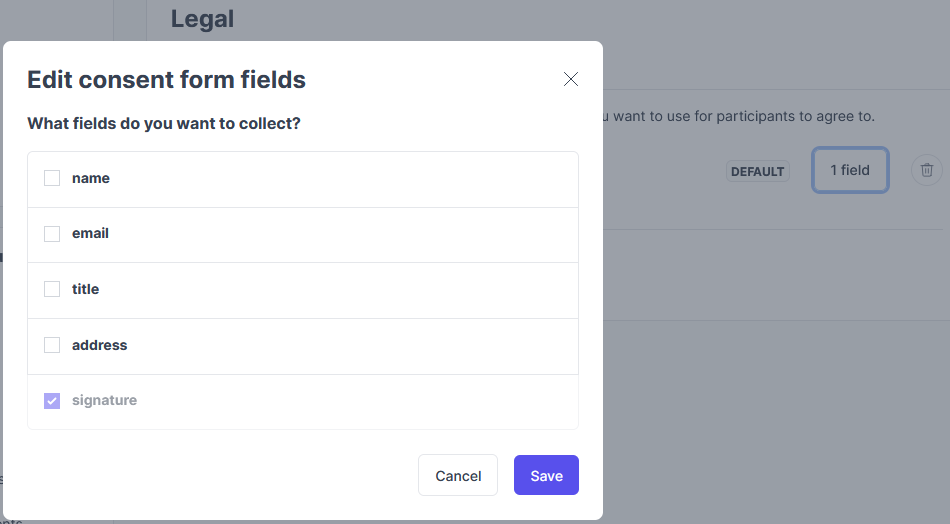
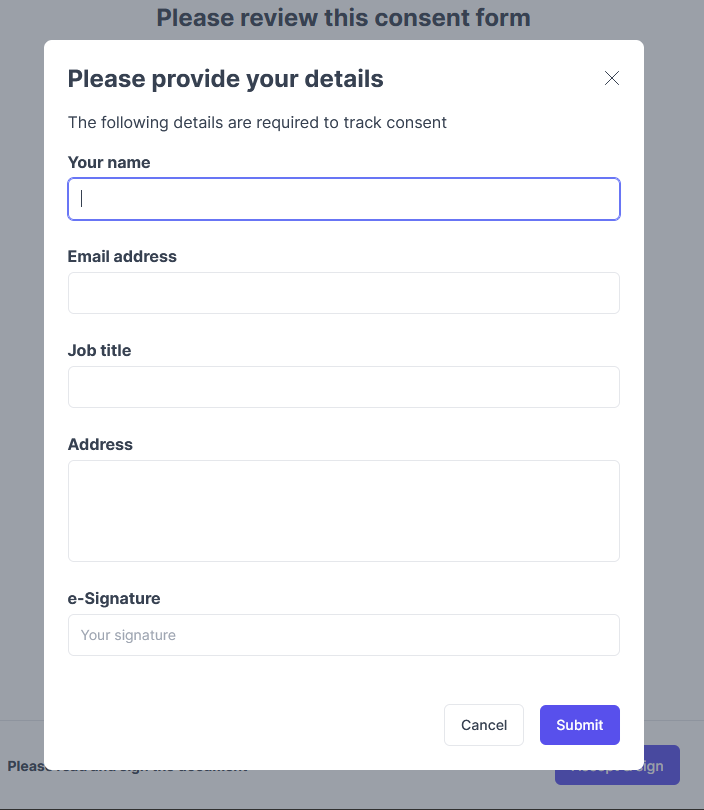
- Optional: Add other fields that you want required for your participants to fill out:
- Name, email, job title, and/or address. Signature is always required.
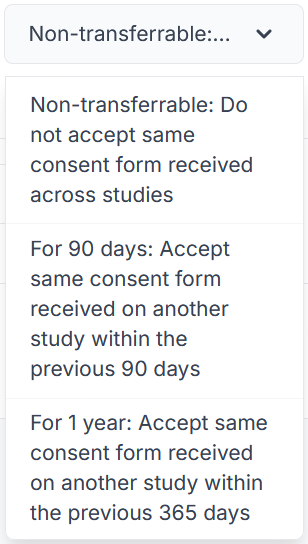
Consent Transfer Settings
Choose if your participants need to sign the same consent form again for each study, or if once is enough.
- Non-transferrable - Do not accept same consent form received across studies
- The participant will have to sign the same form on every study that they participate in, if the same form is applied.
- For 90 days - Accept same consent form received on another study within the previous 90 days
- If a participant signs a consent form on one study, they will not have to sign it again for any future studies for the next 90 days.
- For 1 year - Accept same consent form received on another study within the previous 365 days
- If a participant signs a consent form on one study, they will not have to sign it again for any future studies for the next 365 days.
What does this look like for the participant?

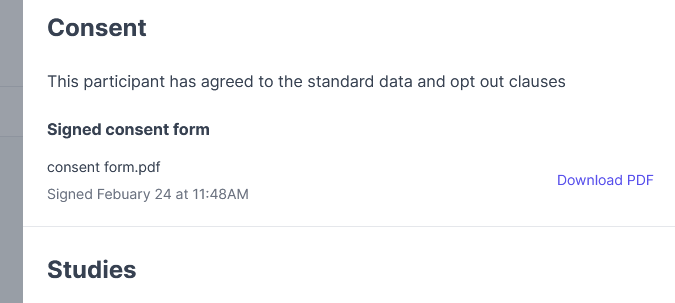
Where can I find the signed consent form?
- You can access the participant's profile and look for Consent, just above Studies.
- Click Download PDF.
Consent form FAQ
What happens if a candidate has already signed the same consent form on a previous study?
- For every study that has a consent from, the candidate will be required to view and offer a digital signature before they can proceed with the study.
Does the participant receive a copy of the signed consent form?
- Not at this time. A copy would need to be downloaded from their profile and sent to them.
Have Questions?
Please reach out to us in the chat or at support@greatquestion.co!